29 September 2023
Get involved: COACH trial
Comparing cochlear implants to hearing aids for adults just outside NICE guidance TA566
by Dr Catherine Killan, on behalf of the COACH trial team
What is current cochlear implant practice?
Cochlear implants (CIs) are well established as a safe, cost-effective hearing intervention for adults with severe or profound deafness who do not gain adequate benefit from acoustic hearing aids (HAs) [1]. They can improve hearing, mental health, and aspects of cognitive function in eligible candidates [2-4]. There is no upper age limit and few health restrictions to receiving a CI, as the procedure can be carried out under either general or local anaesthetic [5]. Under National Institute for Health and Care Excellence (NICE) guidance TA566, private hearing aid dispensers, along with NHS colleagues in audiology and ENT, are responsible for offering referral to a CI service for adults with sensorineural hearing loss where at least two unaided thresholds including 0.5, 1, 2, 3 or 4 kHz are ≥ 80 dB HL in both ears [6]. At the CI service, adults undergo a multidisciplinary assessment including medical checks, hearing therapy, expectation setting, and speech perception assessment, to determine if implantation is the best option for them. If you are interested to find out more, there are lots of resources at the British Cochlear Implant Group website: www.bcig.org.uk.
What is the COACH trial and why is it important?
- There is concern among clinicians and patients that some adults who do not meet NICE guidance TA566 (2019) struggle to hear well enough with HAs.
- However, there is a lack of high quality evidence to base candidacy guidance on. Gold standard evidence is normally from randomised, controlled, clinical trials (RCTs) but to date there has not been an RCT to inform adult CI candidacy [4].
- COACH is at the forefront of CI research because it is a registered RCT comparing the effectiveness of unilateral CI, or unilateral CI with contralateral HA, to HA(s) alone for adults just outside TA566. IRAS Project ID: 297574. ISRCTN: 15352106.
- The inclusion criteria are based on data collected by the British Cochlear Implant Group working party on candidacy, to target adults where there is acceptable uncertainty as to whether CI or HAs would be best. The study is powered to detect clinically meaningful changes in speech perception.
- COACH was designed and is run jointly by hearing researchers at the University of Nottingham, UK, and National Acoustic Laboratories, Australia, Nottingham Clinical Trials Unit, NHS partners, and a patient advisory group.
- We have recruited 35 of our target 130 participants but at a rate slower than expected. The faster we recruit, the faster we can inform guidance and bring clarity to how best to help adults with hearing loss.
Who are we looking for?
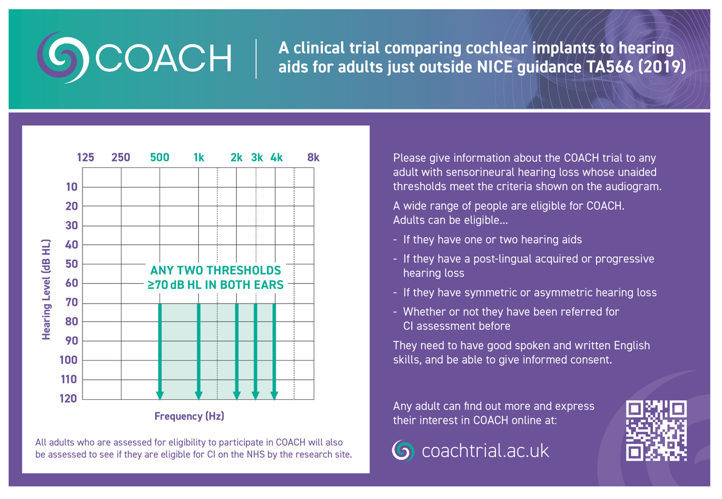
The shaded audiogram and text in Figure 1 explains who is suitable to be given information about the COACH trial.
Figure 1: COACH Trial Referral Criteria
What can you do to help?
Please direct your clients who meet those criteria to our expression of interest form, so they can find out more and identify themselves to us if they wish. This does not commit them to take part. They can submit the expression of interest form online via our website or complete a paper copy (available from our team at [email protected]) and post it to us. If they have a smart phone, you could help them scan our QR code which will take them straight to the webpage where they can open the expression of interest form.
That's all you need to do. The HA user would then have entered our participant pathway and all the other stages including pre-screening onward are completed by our research staff at the research sites.
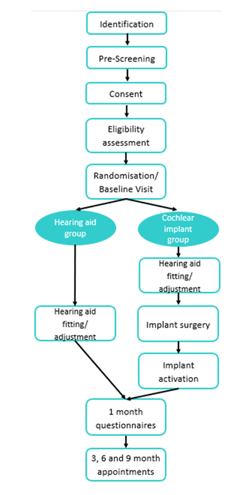
Figure 2: Participant Pathway
COACH participant pathway
- Figure 2 shows an overview of our participant pathway. Adults are identified when they complete an expression of interest form, online or on paper.
- Every adult assessed for eligibility to take part in COACH is also assessed for eligibility for a CI under TA566. Adults within TA566 are offered implantation on the NHS if deemed appropriate by the CI team at the research site.
- Prior to randomisation, participants are checked against eligibility criteria that include speech perception.
- Eligible participants who consent to take part are randomised by a computer algorithm 1:1 to the CI or HA arms.
- HA arm participants are offered new HAs if wanted. CI arm participants are implanted with a standard length electrode array and fitted with a speech processor, plus a new HA for the other ear if appropriate, or they too can choose to keep using their current HA if preferred.
- The primary outcome measure is the change in word test phoneme perception from baseline to 9 months post-intervention. Tests are videoed and scored by an independent assessor who is blinded to intervention and test interval, to minimise assessor bias. Lots of secondary outcome measures are also recorded, including sentence perception in quiet and noise, self-reported hearing, tinnitus, fatigue, and quality of life measures, and a qualitative sub-study where participants are interviewed for an in-depth understanding of their experiences.
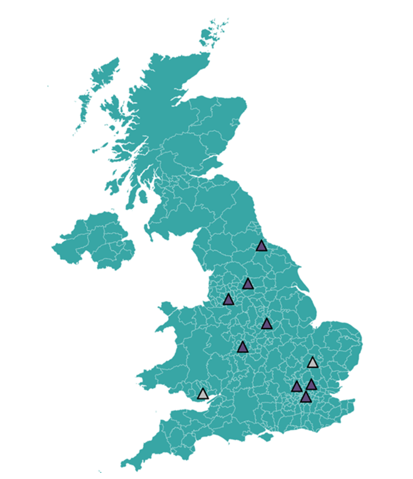
Where are the research sites?
We have sites open and recruiting now at: Bradford Teaching Hospitals NHS Foundation Trust; Guy's and St Thomas' NHS Foundation Trust; Manchester University NHS Foundation Trust; Nottingham University Hospitals NHS Foundation Trust; South Tees Hospitals NHS Foundation Trust; St George's University Hospitals NHS Foundation Trust; University College London Hospitals NHS Foundation Trust; and University Hospitals Birmingham NHS Foundation Trust.
Our site at Cardiff is currently on hold but we hope will reopen, and Cambridge is in site set-up. If you practice in those areas watch out for updates on sites opening, or ask your clients if they would be happy to travel to another available hospital.
Figure 3: Research Sites
Ways you can help your hearing aid users find out more
It can be empowering for people to learn about and take part in healthcare research. As well as accessing specialist clinicians and technology for themselves, it is an opportunity to contribute to the future care and wellbeing of other people around the world.
Here are some simple ways you can help your adult hearing aid users find out more about COACH:
• Read the information for professionals on our website: www.coachtrial.ac.uk
• Invite us to talk to your teams! We can present virtually at your local or regional meetings.
• Get in touch with us at [email protected] to
• Request printed copies of our shaded audiogram prompts to put up in your audiology test rooms, to remind audiologists of the trial referral criteria
• Request patient information leaflets or posters to display in your waiting rooms
• Add COACH participant identification to your team meeting agendas to keep it at the forefront of audiologists' minds
References
[1] Bond et al., "The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: A systematic review and economic model," Health Technology Assessment, vol. 13, no. 44. 2009. doi: 10.3310/hta13440.
[2] Andries et al., "Evolution of Type D Personality Traits After Cochlear Implantation in Severely Hearing Impaired Adults 55 Years and Older: An Exploratory Prospective, Longitudinal, Controlled, Multicenter Study," Otology & Neurotology, vol. 43, no. 8, pp. e865-e871, Sep. 2022, doi: 10.1097/MAO.0000000000003622.
[3] Mertens et al., "Cognitive Improvement After Cochlear Implantation in Older Adults With Severe or Profound Hearing Impairment: A Prospective, Longitudinal, Controlled, Multicenter Study," Ear Hear, vol. 42, no. 3, 2020, doi: 10.1097/AUD.0000000000000962.
[4] Boisvert et al., "Cochlear implantation outcomes in adults: A scoping review," PLoS One, vol. 15, no. 5, p. e0232421, May 2020, doi: 10.1371/journal.pone.0232421.
[5] Walters et al., "Cochlear implantation under local anesthetic: A systematic review and meta-analysis" Laryngoscope Investig Otolaryngol, vol. 7, no. 1, pp. 226-236, Feb. 2022, doi: 10.1002/lio2.720.
[6] National Institute for Health and Care Excellence, "Cochlear implants for children and adults with severe to profound deafness: Technology appraisal guidance [TA566] Recommendations." Accessed: Nov. 30, 2022. [Online]. Available: https://www.nice.org.uk/guidance/TA566/chapter/1-Recommendations

Press enquiries
Media enquiries should be directed to [email protected] or call 020 7298 5110.
We are happy to put you in touch with our expert policy advisers who can comment on a variety of issues.
You can also follow us on Twitter and LinkedIn.

 Your hearing and aural health
Your hearing and aural health  Commissioners and Policymakers
Commissioners and Policymakers  Member support and guidance
Member support and guidance News and views
News and views
 Hearing map
Hearing map